

Carbasugars are analogues of furanoses or pyranoses in which the ring oxygen is replaced by a methylene group. This class of carbohydrate mimetics has attracted increasing interests because of their chemical stability as well as interesting biological properties as antibiotic and as glycosyltransferase and glycosidase inhibitors. However, new naturally occurring carbasugars were rarely reported in recent years.
The research group for Biology of Phytochemical Resources in South China Botanical Garden (SCBG) has obtained a number of antimicrobial fungal strains from the Dinghushan Nature Reserve by microbial isolation and biological activity screening. Rencently, chemical investigation on Ampelomyces sp. SC0307, one of antibacterial fungal strains, was carried out by a Ph.D. candidate ZHANG Huiye under Prof. WEI Xiaoyi’s guidance. As a result, seven new carbasugars, ampelomins AG (Fig. 1), were isolated from the mycelial solid culture of this fungus. Based on analysis of their chemical relation, a unique tetraketide biogenetic pathway was proposed for these compounds and, furthermore, their inhibitory activity against α-glucosidase was evaluated. It was found that their activity depended on the orientation of 2-O-substituent in them. This finding is significant in better understanding of SARs regarding α-glucosidase inhibition of carbasugars and structure optimization of carbasugars for new anti-diabetic agents.
This work was recently published in Journal of Natural Products.
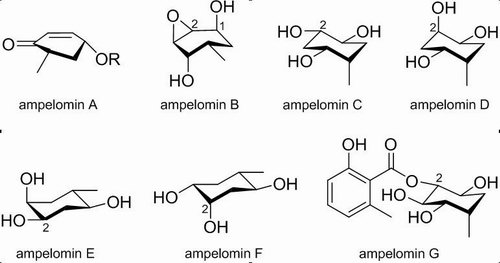
Fig. 1. Chemical structures of ampelomins A-G

